Introduction:
Richter's syndrome (RS) represents transformation of chronic lymphocytic leukemia (CLL) into a highly aggressive lymphoma with dismal prognosis. Transcriptomic alterations have been described in CLL but most studies focused on peripheral blood samples with minimal data on RS-involved tissue. Moreover, transcriptomic features of RS have not been well defined in the era of CLL novel therapies. In this study we investigated transcriptomic profiles of CLL/RS-involved nodal tissue using samples from a clinical trial cohort of refractory CLL and RS patients treated with Pembrolizumab (NCT02332980).
Methods:
Nodal samples from 9 RS and 4 CLL patients in MC1485 trial cohort were reviewed and classified as previously published (Ding et al, Blood 2017). All samples were collected prior to Pembrolizumab treatment. Targeted gene expression profiling of 789 immune-related genes were performed on FFPE nodal samples using Nanostring nCounter® Analysis System (NanoString Technologies, Seattle, WA). Differential expression analysis was performed using NanoStringDiff. Genes with 2 fold-change in expression with a false-discovery rate less than 5% were considered differentially expressed.
Results:
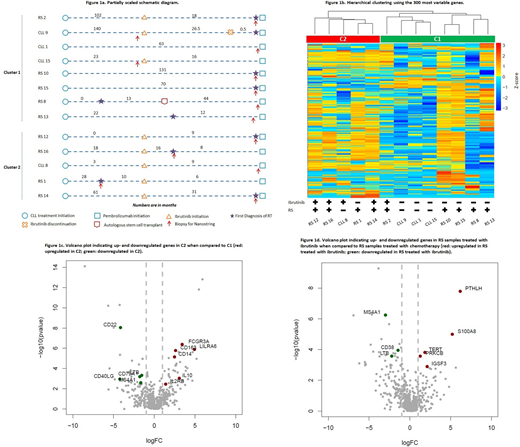
The details for the therapy history of this cohort were illustrated in Figure 1a. All patients exposed to prior ibrutinib before the tissue biopsy had developed clinical progression while receiving ibrutinib. Unsupervised hierarchical clustering using the 300 most variable genes in expression revealed two clusters: C1 and C2 (Figure 1b). C1 included 4 RS and 3 CLL treated with prior chemotherapy without prior ibrutinib, and 1 RS treated with prior ibrutinib. C2 included 1 CLL and 3 RS received prior ibrutinib, and 1 RS treated with chemotherapy. The segregation of gene expression profiles in samples was largely driven by recent exposure to ibrutinib. In C1 cluster (majority had no prior ibrutinb), RS and CLL samples were clearly separated into two subgroups (Figure 1b). In C2 cluster, CLL 8 treated with ibrutinib showed more similarity in gene expression to RS, than to other CLL samples treated with chemotherapy.
In comparison of C2 to C1, we identified 71 differentially expressed genes, of which 34 genes were downregulated and 37 were upregulated in C2. Among the upregulated genes in C2 (majority had prior ibrutinib) are known immune modulating genes including LILRA6, FCGR3A, IL-10, CD163, CD14, IL-2RB (figure 1c). Downregulated genes in C2 are involved in B cell activation including CD40LG, CD22, CD79A, MS4A1 (CD20), and LTB, reflecting the expected biological effect of ibrutinib in reducing B cell activation. Among the 9 RS samples, we compared gene profiles between the two groups of RS with or without prior ibrutinib therapy. 38 downregulated genes and 10 upregulated genes were found in the 4 RS treated with ibrutinib in comparison with 5 RS treated with chemotherapy. The top upregulated genes in the ibrutinib-exposed group included PTHLH, S100A8, IGSF3, TERT, and PRKCB, while the downregulated genes in these samples included MS4A1, LTB and CD38 (figure 1d).
In order to delineate the differences of RS vs CLL, we compared gene expression profiles between 5 RS samples and 3 CLL samples that were treated with only chemotherapy. RS samples showed significant upregulation of 129 genes and downregulation of 7 genes. Among the most significantly upregulated genes are multiple genes involved in monocyte and myeloid lineage regulation including TNFSF13, S100A9, FCN1, LGALS2, CD14, FCGR2A, SERPINA1, and LILRB3.
Conclusion:
Our study indicates that ibrutinib-resistant, RS-involved tissues are characterized by downregulation of genes in B cell activation, but with PRKCB and TERT upregulation. Furthermore, RS-involved nodal tissues display the increased expression of genes involved in myeloid/monocytic regulation in comparison with CLL-involved nodal tissues. These findings implicate that differential therapies for RS and CLL patients need to be adopted based on their prior therapy and gene expression signatures. Studies using large sample size will be needed to verify this hypothesis.
Zhao:Merck: Current Employment. Blumenschein:Merck: Current Employment. Yearley:Merck: Current Employment. Wang:Novartis: Research Funding; Incyte: Research Funding; Innocare: Research Funding. Parikh:Verastem Oncology: Honoraria; GlaxoSmithKline: Honoraria; Pharmacyclics: Honoraria, Research Funding; MorphoSys: Research Funding; Ascentage Pharma: Research Funding; Genentech: Honoraria; AbbVie: Honoraria, Research Funding; Merck: Research Funding; TG Therapeutics: Research Funding; AstraZeneca: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Kenderian:Sunesis: Research Funding; MorphoSys: Research Funding; Humanigen: Consultancy, Patents & Royalties, Research Funding; Gilead: Research Funding; BMS: Research Funding; Tolero: Research Funding; Lentigen: Research Funding; Juno: Research Funding; Mettaforge: Patents & Royalties; Torque: Consultancy; Kite: Research Funding; Novartis: Patents & Royalties, Research Funding. Kay:Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; Juno Theraputics: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; MEI Pharma: Research Funding; Agios Pharma: Membership on an entity's Board of Directors or advisory committees; Bristol Meyer Squib: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Cytomx: Membership on an entity's Board of Directors or advisory committees. Braggio:DASA: Consultancy; Bayer: Other: Stock Owner; Acerta Pharma: Research Funding. Ding:DTRM: Research Funding; Astra Zeneca: Research Funding; Abbvie: Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Membership on an entity's Board of Directors or advisory committees; alexion: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal